Embark on a journey of discovery with our meticulously crafted Counting Moles and Atoms Worksheet. Dive into the fascinating world of chemistry as we unveil the intricacies of moles and atoms, exploring their profound significance in various scientific disciplines.
This comprehensive resource provides a solid foundation for understanding the fundamental concepts of mole and atom counting, empowering you with the tools to navigate complex chemical calculations with confidence.
Counting Moles and Atoms: Counting Moles And Atoms Worksheet
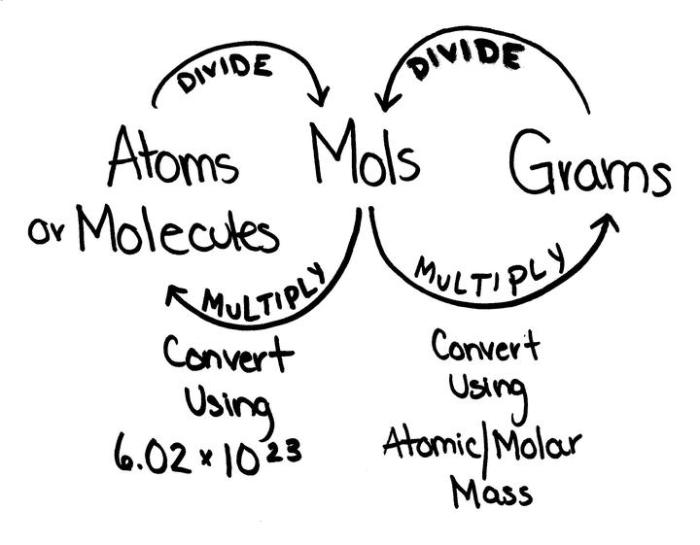
In chemistry, a mole is a unit of measurement used to quantify the amount of a substance. It is defined as the amount of substance that contains exactly 6.022 × 10 23elementary entities, which can be atoms, molecules, ions, or electrons.
This number is known as Avogadro’s number.
The mole concept provides a convenient way to convert between the mass and the number of particles in a sample of a substance. The molar mass of a substance is the mass of one mole of that substance. The molar mass is expressed in grams per mole (g/mol).
Relationship Between Moles and Atoms, Counting moles and atoms worksheet
The relationship between moles and atoms is given by the following equation:
number of atoms = number of moles × Avogadro’s number
For example, if you have 1 mole of carbon, you have 6.022 × 10 23carbon atoms.
Converting Between Moles and Atoms
To convert between moles and atoms, you can use the following steps:
- Convert the mass of the substance to moles by dividing the mass by the molar mass.
- Multiply the number of moles by Avogadro’s number to get the number of atoms.
For example, if you have 12 grams of carbon, you can convert it to moles by dividing by the molar mass of carbon (12 g/mol):
g ÷ 12 g/mol = 1 mol
Then, you can multiply the number of moles by Avogadro’s number to get the number of atoms:
mol × 6.022 × 1023atoms/mol = 6.022 × 10 23atoms
FAQ Resource
What is the significance of understanding mole and atom counting?
Understanding mole and atom counting is crucial for accurately determining the quantities of reactants and products involved in chemical reactions, enabling precise predictions and calculations in various scientific disciplines.
How does the worksheet facilitate the learning process?
The worksheet is designed to provide a structured and interactive learning experience, featuring practice problems, tables for organizing solutions, and sections for demonstrating understanding, promoting active engagement and reinforcement of key concepts.
What are the practical applications of mole and atom counting in real-world scenarios?
Mole and atom counting play a vital role in fields such as chemistry, physics, and engineering, enabling the determination of substance quantities, stoichiometric calculations, and the development of new materials and technologies.
