Delving into the formula for calcium nitrate trihydrate, we embark on a journey to unravel the intricacies of this compound. Its molecular structure, physical and chemical properties, and diverse applications will be meticulously explored, providing a comprehensive understanding of its significance in various industries.
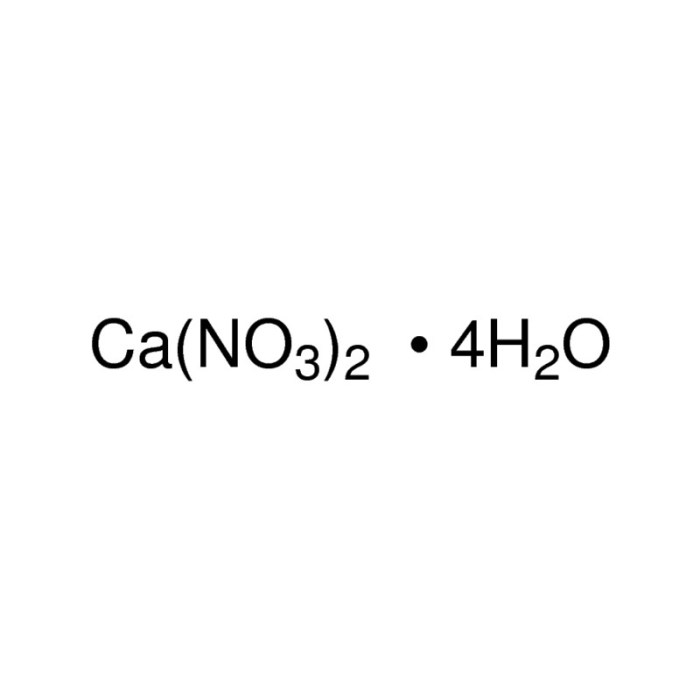
Calcium nitrate trihydrate, with its unique formula Ca(NO3)2·3H2O, possesses a crystalline structure that plays a crucial role in its properties and applications. Its physical characteristics, including color, odor, density, and solubility, will be thoroughly examined, shedding light on its behavior in different environments.
Chemical Composition and Structure
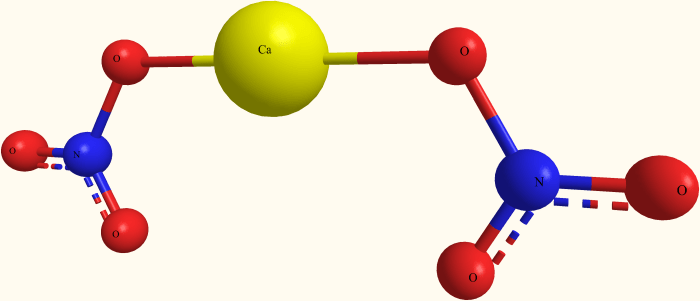
Calcium nitrate trihydrate is an inorganic compound with the molecular formula Ca(NO 3) 2·3H 2O. It is composed of calcium ions (Ca 2+), nitrate ions (NO 3–), and water molecules (H 2O).
The crystal structure of calcium nitrate trihydrate is monoclinic. The calcium ions are coordinated by six water molecules, forming octahedral complexes. The nitrate ions are located between the calcium ions, forming a network of hydrogen bonds.
Physical and Chemical Properties
Calcium nitrate trihydrate is a white or colorless solid with a density of 1.82 g/cm 3. It is odorless and has a bitter taste. It is soluble in water and slightly soluble in alcohol.
Calcium nitrate trihydrate is a stable compound, but it can decompose when heated. It is also hygroscopic, meaning that it absorbs moisture from the air.
Production and Synthesis
Calcium nitrate trihydrate is produced by reacting calcium carbonate (CaCO 3) with nitric acid (HNO 3). The reaction is carried out in a solution, and the calcium nitrate trihydrate crystallizes out of the solution.
The reaction can be represented by the following equation:
CaCO3+ 2HNO 3→ Ca(NO 3) 2+ H 2O + CO 2
Applications and Uses
Calcium nitrate trihydrate is used in a variety of applications, including:
- As a fertilizer in agriculture
- As a component of concrete and other building materials
- As a flux in soldering and welding
- As a preservative in food
- As a source of calcium in dietary supplements
Safety and Handling

Calcium nitrate trihydrate is a non-toxic compound, but it can cause irritation to the skin, eyes, and respiratory tract. It is also a fire hazard, and it can explode if it is heated rapidly.
When handling calcium nitrate trihydrate, it is important to wear gloves, eye protection, and a dust mask. It is also important to store calcium nitrate trihydrate in a cool, dry place away from heat and open flames.
Environmental Impact: Formula For Calcium Nitrate Trihydrate

Calcium nitrate trihydrate is a relatively environmentally friendly compound. It is biodegradable and does not accumulate in the environment.
However, calcium nitrate trihydrate can contribute to water pollution if it is not used properly. When it is applied to soil, it can leach into groundwater and contaminate drinking water supplies.
Question & Answer Hub
What is the molecular formula for calcium nitrate trihydrate?
Ca(NO3)2·3H2O
What is the color of calcium nitrate trihydrate?
White
What is the density of calcium nitrate trihydrate?
1.82 g/cm³
What are the applications of calcium nitrate trihydrate?
Fertilizer, concrete additive, deicing agent