Uranium-232 has a half life of 68.8 years – Uranium-232, a radioactive isotope with a half-life of 68.8 years, plays a crucial role in various industries and scientific applications. Its unique properties and decay characteristics make it an intriguing subject of study, with implications for nuclear energy, medicine, and environmental science.
This comprehensive overview delves into the significance of uranium-232, exploring its half-life, applications, decay chain, safety considerations, and environmental impact. Prepare to embark on an enlightening journey into the world of radioactive isotopes and their multifaceted roles.
Introduction
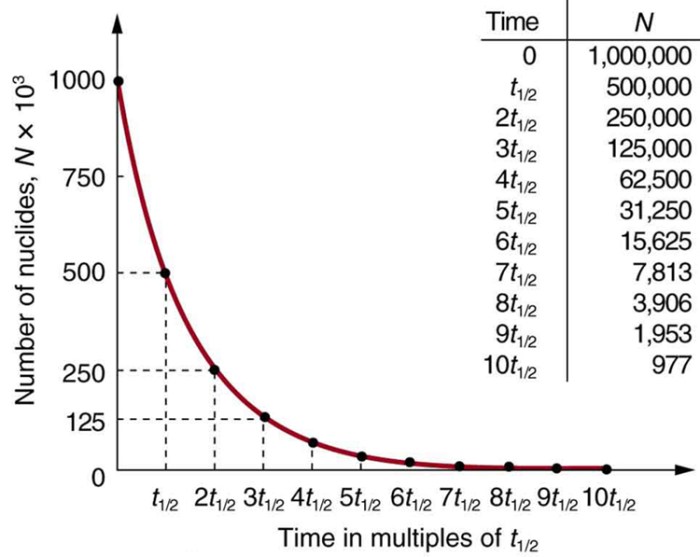
Uranium-232 is a naturally occurring radioactive isotope of uranium. It has a relatively long half-life of 68.8 years, making it a useful material for a variety of applications in nuclear energy, medicine, and other fields.
Half-Life of Uranium-232
Half-life is a measure of the time it takes for half of the atoms in a radioactive sample to decay. The half-life of uranium-232 is 68.8 years. This means that after 68.8 years, half of the uranium-232 atoms in a sample will have decayed into other elements.
Applications of Uranium-232
Uranium-232 has a number of important applications, including:
- Nuclear energy: Uranium-232 is used as a fuel in nuclear reactors.
- Medicine: Uranium-232 is used in the production of medical isotopes, such as technetium-99m, which is used in medical imaging.
- Other applications: Uranium-232 is also used in a variety of other applications, such as neutron sources and radiation shielding.
Decay Chain of Uranium-232: Uranium-232 Has A Half Life Of 68.8 Years

Uranium-232 decays through a series of alpha and beta decays into the stable isotope lead- 208. The following table shows the decay chain of uranium-232:
| Isotope | Half-Life | Type of Decay |
|---|---|---|
| Uranium-232 | 68.8 years | Alpha decay |
| Thorium-228 | 1.9 years | Beta decay |
| Radium-224 | 3.6 days | Alpha decay |
| Radon-220 | 55.6 seconds | Alpha decay |
| Polonium-216 | 0.15 seconds | Alpha decay |
| Lead-212 | 10.6 hours | Beta decay |
| Bismuth-212 | 60.6 minutes | Alpha decay |
| Polonium-212 | 0.3 microseconds | Alpha decay |
| Lead-208 | Stable | – |
Safety Considerations
Uranium-232 is a radioactive material and must be handled with care. The following safety precautions should be taken when handling uranium-232:
- Use proper personal protective equipment, such as gloves, a lab coat, and a respirator.
- Work in a well-ventilated area.
- Keep uranium-232 away from food and drink.
- Dispose of uranium-232 properly according to local regulations.
Environmental Impact
Uranium-232 and its decay products can have a negative impact on the environment. The following are some of the potential environmental impacts of uranium-232:
- Radiation exposure: Uranium-232 and its decay products can emit radiation, which can be harmful to human health and the environment.
- Groundwater contamination: Uranium-232 and its decay products can leach into groundwater, contaminating drinking water supplies.
- Soil contamination: Uranium-232 and its decay products can contaminate soil, making it unsuitable for growing crops or grazing animals.
Questions Often Asked
What is the significance of uranium-232’s 68.8-year half-life?
The 68.8-year half-life of uranium-232 determines its rate of radioactive decay, which influences its applications and safety considerations.
What are the primary applications of uranium-232?
Uranium-232 finds applications in nuclear energy as a fertile material, in medicine for targeted alpha therapy, and in various industrial processes.
How does uranium-232 decay?
Uranium-232 undergoes alpha decay, emitting alpha particles and transforming into thorium-228.