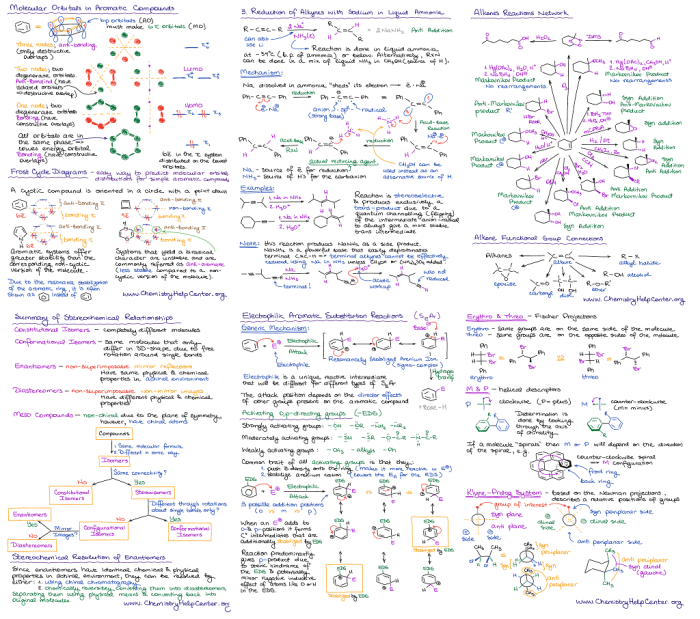
Welcome to your ultimate organic chem 2 cheat sheet, a treasure trove of knowledge that will empower you to conquer the complexities of this fascinating subject. From functional groups to reactions, spectroscopy to synthesis, this guide has got you covered.
Prepare to embark on an exciting journey filled with clarity, understanding, and a touch of fun.
In the realm of organic chemistry, functional groups reign supreme. They are the building blocks of molecules, each with its unique personality and reactivity. Our cheat sheet will introduce you to the A-team of functional groups, revealing their properties and how they interact with each other.
But that’s just the beginning!
Functional Groups
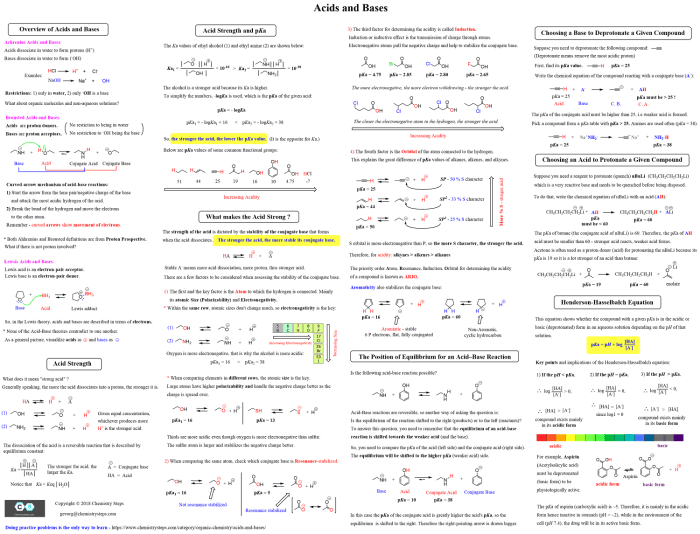
Functional groups are atoms or groups of atoms that impart characteristic chemical properties to organic molecules. They determine the reactivity and physical properties of the compounds they are attached to.
The following table summarizes the common functional groups encountered in organic chemistry 2, along with their structures and general reactivity:
| Functional Group | Structure | Reactivity |
|---|---|---|
| Alkanes | C-H | Unreactive |
| Alkenes | C=C | Reactive, undergo addition reactions |
| Alkynes | C≡C | More reactive than alkenes, undergo addition reactions |
| Alcohols | R-OH | React with acids to form esters, undergo oxidation |
| Ethers | R-O-R | Unreactive, except under acidic conditions |
| Aldehydes | R-CHO | React with nucleophiles to form addition products, undergo oxidation |
| Ketones | R-CO-R | React with nucleophiles to form addition products, undergo reduction |
| Carboxylic Acids | R-COOH | React with bases to form salts, undergo esterification |
| Esters | R-COOR | React with bases to form salts, undergo hydrolysis |
| Amides | R-CONH2 | React with acids to form salts, undergo hydrolysis |
| Nitriles | R-CN | React with nucleophiles to form addition products, undergo hydrolysis |
| Amines | R-NH2 | React with acids to form salts, undergo alkylation |
Reactions

Organic chemistry 2 involves a wide range of reactions that can be broadly classified into three main types: nucleophilic substitution, electrophilic addition, and elimination reactions.
Nucleophilic Substitution Reactions, Organic chem 2 cheat sheet
Nucleophilic substitution reactions occur when a nucleophile (an electron-rich species) attacks an electrophile (an electron-poor species), resulting in the substitution of one group by another. The general mechanism involves the nucleophile attacking the electrophile, forming a new bond and breaking the old bond between the electrophile and the leaving group.
For those struggling with Organic Chemistry 2, a comprehensive cheat sheet can be a lifesaver. Similarly, if you’re facing the CPS Algebra Exit Exam in 2023, it’s crucial to prepare thoroughly. With ample resources available, like this guide , you can confidently tackle the exam.
Returning to organic chemistry, the cheat sheet can provide quick access to essential formulas and reactions, making studying more efficient and effective.
Examples of nucleophilic substitution reactions include:
- SN2 reactions: These reactions occur in a single step, where the nucleophile attacks the electrophile simultaneously with the departure of the leaving group.
- SN1 reactions: These reactions occur in two steps, where the electrophile first ionizes to form a carbocation, which is then attacked by the nucleophile.
Electrophilic Addition Reactions
Electrophilic addition reactions occur when an electrophile adds to a double or triple bond. The general mechanism involves the electrophile attacking the double or triple bond, forming a new bond between the electrophile and each of the carbon atoms in the bond.
Examples of electrophilic addition reactions include:
- Addition of hydrogen halides (HX) to alkenes
- Addition of water to alkenes
- Addition of halogens (X 2) to alkenes
Elimination Reactions
Elimination reactions occur when a small molecule (often water or hydrogen halide) is removed from a molecule, resulting in the formation of a double or triple bond. The general mechanism involves the removal of a proton from a carbon atom adjacent to the double or triple bond, followed by the removal of a leaving group from the other carbon atom.
Examples of elimination reactions include:
- E2 reactions: These reactions occur in a single step, where the proton and the leaving group are removed simultaneously.
- E1 reactions: These reactions occur in two steps, where the proton is first removed to form a carbocation, which is then deprotonated to form the double or triple bond.
| Reaction Type | Mechanism | Products |
|---|---|---|
| Nucleophilic Substitution (SN2) | One-step attack of nucleophile on electrophile | Substitution of leaving group by nucleophile |
| Nucleophilic Substitution (SN1) | Two-step ionization of electrophile followed by attack of nucleophile | Substitution of leaving group by nucleophile |
| Electrophilic Addition | Attack of electrophile on double or triple bond | Addition of electrophile to each carbon in the bond |
| Elimination (E2) | One-step removal of proton and leaving group | Formation of double or triple bond |
| Elimination (E1) | Two-step removal of proton to form carbocation followed by deprotonation | Formation of double or triple bond |
Spectroscopy

Spectroscopy is a powerful tool for identifying and characterizing organic compounds. It involves the interaction of electromagnetic radiation with molecules, causing them to absorb or emit energy at specific wavelengths. By analyzing these interactions, we can determine the structure and functional groups present in a molecule.
Infrared Spectroscopy (IR)
IR spectroscopy measures the absorption of infrared radiation by a molecule. Functional groups with strong dipoles, such as C=O, C-N, and O-H, absorb IR radiation at characteristic frequencies. By matching the observed IR spectrum to a library of known spectra, we can identify the functional groups present.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy measures the magnetic properties of certain atomic nuclei, such as 1H and 13C. Different types of atoms and their environments within a molecule give rise to distinct NMR signals. By analyzing the chemical shifts, splitting patterns, and coupling constants, we can determine the structure and connectivity of a molecule.
Mass Spectrometry (MS)
MS involves ionizing a molecule and measuring the mass-to-charge ratio of the resulting ions. This provides information about the molecular weight and elemental composition of the molecule. MS can also be used to identify fragments of a molecule, which can help in determining its structure.
Solving Structural Problems
Spectroscopy is essential for solving structural problems in organic chemistry. By combining the information obtained from different spectroscopic techniques, we can determine the structure of unknown compounds, identify impurities, and elucidate reaction mechanisms.
Synthesis
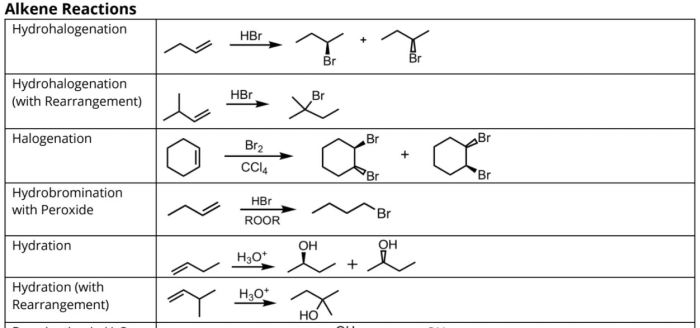
Organic synthesis is the process of creating organic compounds from simpler starting materials. It’s a fundamental skill in organic chemistry and has applications in various fields, including pharmaceuticals, materials science, and food chemistry.
The general principles of organic synthesis involve:
- Identifying the target molecule and its functional groups.
- Selecting appropriate starting materials and reagents.
- Applying specific reactions to transform the starting materials into the target molecule.
- Optimizing reaction conditions to maximize yield and selectivity.
- Purifying the final product.
Methods for Constructing Carbon-Carbon Bonds
One of the key aspects of organic synthesis is the formation of carbon-carbon bonds. Several methods can be used for this purpose, including:
- Alkylation:The addition of an alkyl group to a carbon atom. Common alkylating agents include alkyl halides, Grignard reagents, and organolithium compounds.
- Acylation:The addition of an acyl group (RCO-) to a carbon atom. Acylating agents include acid chlorides, anhydrides, and esters.
- Condensation reactions:The formation of a new carbon-carbon bond by the elimination of a small molecule, such as water or alcohol. Common condensation reactions include aldol condensations, Claisen condensations, and Knoevenagel condensations.
Flowchart of Organic Synthesis
The following flowchart summarizes the general steps involved in organic synthesis:
- Identify the target molecule.
- Design a synthetic pathway.
- Select appropriate starting materials and reagents.
- Carry out the reactions under optimized conditions.
- Purify the final product.
- Analyze the product to confirm its structure and purity.
FAQ Guide: Organic Chem 2 Cheat Sheet
What is the most important functional group in organic chemistry?
The carbonyl group (C=O) is widely regarded as the most important functional group due to its versatility and prevalence in organic molecules.
How can I identify organic compounds using spectroscopy?
Spectroscopic techniques such as IR, NMR, and mass spectrometry provide valuable information about the structure and composition of organic compounds. By analyzing the characteristic peaks and signals in the spectra, you can identify and characterize unknown compounds.
What is the key to successful organic synthesis?
The key to successful organic synthesis lies in understanding the reactivity of functional groups and the mechanisms of organic reactions. By carefully planning the sequence of reactions and selecting the appropriate reagents, you can achieve the desired target molecule efficiently.